Optimizing energy storage
 After running my solar power system for a while, and planning the heating system, it became clear to me that energy storage is the hardest part to solve.
After running my solar power system for a while, and planning the heating system, it became clear to me that energy storage is the hardest part to solve.
The electrical batteries are the weakest link of the system. Unless you spend a lot of money on a large battery bank, you will have to be thrifty with your consumption, and you constantly worry about the charge level.
There are three paths you can take in this:
A) Minimize the consumption of electricity. Run the fridge on propane. Rewire the house to use 12 V for lights and appliances to minimize the power loss in the inverter. For appliances that require 230 VAC, use a high efficiency power inverter, only turned on when in use.
B) Invest in a very large battery bank. Get 30 batteries and stop worrying. Do additional charging with a gasoline powered generator in the winter when solar panels produce too little. However, since batteries are worn out over time, you either have to accept the cost of replacing them every ten years, or learn how to refurbish batteries, which is a messy toxic chemistry.
C) Find some alternative way to store energy. This must be cost effective, and convert easily to electricity. I decided to take some time and explore it in this document.
Energy types
To understand the challenge, it helps to take a look at different kinds of energy. All machines have an energy source, and this energy is converted to some other forms of energy, as the machine performs something useful. For example a toaster uses electricity to heat filaments which toasts the bread.
- Electrical energy. The most useful form of energy. Converts easily to other forms, for example in electric motors to provide mechanical force, or in radiators to provide heat. However there is not yet any practical ways to store electricity directly, although in the future super conductivity might do the trick.
- Potential energy. Energy through gravity, released when a heavy object is falling from an elevated level to a lower level. It's a very simple form of energy with long historical use and that doesn't require complex machines, but that has relatively low energy density. For example water in an elevated dam. It could also be energy through pressure, for example a pressurized gas in a container, or energy through preloaded mechanical devices such as a spring.
- Kinetic energy. Energy through momentum, such as an object that is moving at a speed, for example the wind blowing, a running stream of water, waves on the ocean, or a spinning wheel. This is together with potential energy are the two forms of "mechanical energy".
- Chemical energy. Energy stored in chemical bonds, for example in batteries, gasoline or firewood. Changing the bonds transforms it into a different material, releasing energy at the same time. For example an internal combustion engine transforms chemical energy (gasoline) into heat and potential energy (cylinder pressure), which is then converted to kinetic energy moving the car forward. Some chemical energy sources are extremely compact and useful.
- Radiation energy. Energy through sun rays. A practically unlimited form of energy, however relatively low in density, and unevenly available.
- Heat energy. The innate energy in matter at a certain temperature. Very high energy density and easy storage, and can be converted to other forms, by the use of a heat engine that exploits temperature differences. However it is the energy with the highest entropy, so it is the hardest to convert to other energy forms.
Consuming the energy at the moment it is naturally available is of course the easiest way to do it, for example letting the sun rays heat up your home, or letting the rain fall water your tomatoes. But in most scenarios we can't wait around for things to happen naturally, so an energy storage system must be used, where energy first is harvested, then stored, and then consumed. In this system the energy is also transformed between the stages, for example solar panels (radiation energy), which charges batteries (chemical energy), which runs a water pump (electrical energy and mechanical energy).
Energy sources
 Since I want a self sustainable lifestyle in my cottage, I need to use free energy sources, which limits me to:
Since I want a self sustainable lifestyle in my cottage, I need to use free energy sources, which limits me to:
- Firewood. Easy to harvest, high energy density.
- Heating from the sun. Compared to firewood a very low energy, and only usable on sunny days, so I can't rely on this.
- Rain / rivers. I have a small stream on the property, but the water flow is too small for it to be useful.
- Sun radiation. I already have eight solar panels installed, which give me up to 2 kW of power in full sunshine.
- Wind power. It is quite windy, so it could work well to complement my solar panels. However, a wind turbine that gives any reasonable output is quite large in size, which makes me hesitate.
Energy consumption
My cottage has a power need for heating of 2 kW for the winter months, and 1 kW for the spring and fall months. I exclude the cooking stove from this calculation, which I run separately on propane. In total the heating needs are 8,640 kWh per year. This is the equivalent of burning 4 m3f of birch firewood. It's not a very high volume, easily collected during a few weeks of spring.
The electricity consumption is 900 kWh per year. With an estimated 850 sun-hours per year, my 2 kW solar panels produce a total of 1700 kWh/year, more than enough for my needs. But the problem is not production, it is storage in the batteries, as mentioned above. The battery bank would have to be really large for me to feel comfortable, and I would still have to replace the batteries periodically.
The heating and electricity can be separate systems, which is the most common way. But since the electricity consumption is only a fraction of the heating, I will also explore ways to combine them into a single system by generating electricity as a by-product of heating, sometimes called cogeneration.
Energy storage
Below I will look at a few different ways to store energy, and rate them according to usefulness. The parameters to look at when selecting an energy storage system are:
- Energy density (so there is enough space to implement it)
- Cost of implementation and maintenance
- How to convert the stored energy into electricity
To compare with my current battery storage, it is ten 12 V, 100 Ah batteries, which in theory gives me 12 kWh. But since their lifespan is dependent on discharge level not getting too low, a more realistic value is 4 kWh. With a total consumption of 900 kWh per year, it would be more practical to have at least two weeks of energy reservoir, which gives a target of 35 kWh for the examples below (almost tenfold over my current batteries).
Elevated water pond
The official name is pumped-storage hydroelectricity. Since I have a 10 m tall hill above my cottage, and a 10 m slope down to my well, I effectively have 20 m of elevation to use for some kind of potential energy storage system.
Pipes could run down the hill, and at the bottom would be a water turbine connected to a generator that produces electricity. The used water runs into a lower pond, and is pumped up again when there is sunshine to power it through the solar panels.
Potential energy is calculated through: E = m * g * h (m = mass in kilograms, g = the gravity constant of 9.82, h = height in meters).
Let's say I dig a pond that is 10 x 10 x 2 meters on my hill, and also use it for fish farming. It would be a beautiful addition to the property. Limiting the use of half of the water for energy storage, I get: E = 10 * 10 * 1 * 1000 * 9.82 * 20 = 5.5 kWh (19.6 MJ). This pond is already very large, but it would have to be seven times that volume to reach the target of 35 kWh, which is not realistic.
Conclusion: Not enough space, too expensive.
Below: the pumped storage facility at Goldisthal, Germany.

Railway with a heavy rail car
The idea is to build a railway from the bottom to the top of the hill. At the top is a winch with a motor that can also act as a generator. At sunshine the solar panels power the motor to slowly pull the car uphill. When electricity is needed the car is let rolling downhill to turn the generator.
If I get hold of a standard 100 ton freight car, then I would get the same result as with the pond above: 5.5 kWh. This car would be 18 m long, so I doubt more than one would be practical on such a short track. The cost would be really high.
Conclusion: Not enough space, too expensive.
Below: The Ares project of energy storage through gravity pulled rail cars.

Pressurized air tank
 A compressor is pumping air inside a large tank, and it lets air out through a turbine to generate electricity when needed.
A compressor is pumping air inside a large tank, and it lets air out through a turbine to generate electricity when needed.
I could get a large pressure vessel, for example a cylindrical steel tank with a connected compressor. A 3 m long carbon steel tank with 1,5 m diameter and 12 mm thickness, has a volume of 5.3 m3 and can hold a pressure of 300 PSI (2 MPa). Under ideal conditions, the energy stored would be 4.2 kWh (15 MJ). I would have to get eight or more such tanks to reach my goal, which is not realistic. Also, the efficiency round trip is low for this storage system, because the way gases heat up and cool down during compression and expansion. And this is essentially a "bomb" waiting to explode, so it has to be dug down into the ground for safety.
Conclusion: Not practical, too expensive.
Fly-wheel
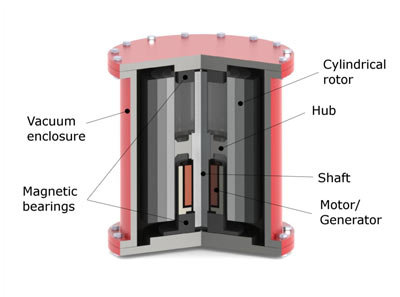 A balanced heavy wheel spins at high speed on high quality bearings. On the fly-wheel is an electrical motor, that can also act as a generator.
A balanced heavy wheel spins at high speed on high quality bearings. On the fly-wheel is an electrical motor, that can also act as a generator.
For a disc-shaped flywheel, the stored energy is calculated through:
E = m * d ^ 2 * rpm ^ 2 / 1459, (m = mass in kg, d = diameter of the disc in m, rpm = revolutions per minute).
As can be seen in this formula, the kinetic energy improved the most by increasing the diameter or spinning speed. Because of the centrifugal forces, the fly-wheel material tensile strength sets the upper limit for the design.
Example of a large flywheel: 2 m in diameter, 1000 kg, 1000 rpm: 0.75 kWh (2.7 MJ).
Example of a small and very fast fly-wheel, NASA technology: 0.7 m in diameter, 25 kg, 70,000 rpm: 11 kWh (41 MJ).
A fly-wheel has to be very well balanced, since even the smallest vibration could wear out the bearings prematurely. It contains so much energy it has be buried in the ground in case it loses balance and crashes.
Conclusion: Not practical, unless you use expensive NASA technology.
Hydrogen fuel cell
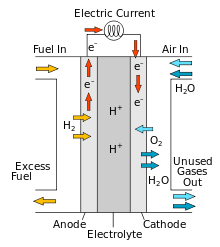 A hydrogen storage system that uses the solar panel power for electrolysis of water, which creates hydrogen gas that is pumped into a tank. When power is needed the hydrogen combines with oxygen from air in a fuel cell to produce electricity.
A hydrogen storage system that uses the solar panel power for electrolysis of water, which creates hydrogen gas that is pumped into a tank. When power is needed the hydrogen combines with oxygen from air in a fuel cell to produce electricity.
This technology has not caught on yet because hydrogen is complicated to store, parts are expensive, and the system has low efficiency. It only returns 30% of the electricity put into it.
One liter of hydrogen at 700 bar pressure has 1.6 kWh (5.6 MJ) of chemical energy. Using a more modest compression of 300 bar, a 100 L tank would contain 68 kWh of energy, out of which approx 50% (34 kWh) would reach appliances. This would be enough for my needs.
This is still expensive technology though. A 3 kW fuel cell costs around $20,000, and I haven't managed to find parts from second hand hydrogen cars. Perhaps in the future, prices will come down. The redux flow battery is a similar technology that does away with some complexity, but is unfortunately not a mature technology yet.
Conclusion: Might be practical, but too expensive at the moment.
Heated water tank
This is a very common way to store heat when heating homes.
The formula for calculating stored heat is: E = v * sh * dT, (v = volume in liters, sh = specific heat capacity in kJ/L C, dT = temperature difference).
For example, a 500 L tank containing 80 C water in a 20 C room: E = 500 * 4.2 * (80 - 20) = 35 kWh (126 MJ).
That would be precisely what I need. However, heat transforms to electricity in a very inefficient way, so I need to look at different types of heat engines before I decide on this solution (more below). Some tank insulation calculations also have to be done.
Water turns out to be a very good storage medium for heat. It is cheap, non toxic, and with a very good value for specific heat capacity (kJ/L C): Water: 4.2. Concrete: 1.8. Wood: 1.2. Gypsum: 2.4. Soil: 2.1. Steel: 3.8. Aluminum: 2.4. Paraffin: 2.9. Table salt: 1.9.
Conclusion: Practical and safe, but needs efficient heat engine.
Molten salt tank
This method is currently used in large solar power plants, where the sun rays heat certain salts until they melt, which are stored in an insulated chamber until power is needed. Then they heat water to produce steam, which power turbines with electrical generators.
The advantage of using salt over water is that it can be heated to a higher temperature, and that the enthalpy of fusion when the salt melts also adds to the energy content. The heat energy required to raise the temperature of a material is linear until the material is beginning to melt, and then a lot more energy is required for the melting process. The opposite is also true, so when a material gives away heat by cooling down, a lot of extra heat energy is released at the solidification stage.
A useful salt mixture is 60% NaNO3 with 40% KNO3, which melts at 221 C, has a specific heat of 1 kJ/kg C, a heat of fusion of 161 kJ/kg, and a density of 2.2 kg/L.
A 150 L tank of the salt mixture at 250 C in a 20 C room: E = 150 * 2.2 * 1 * (250 - 20) plus 150 * 2.2 * 161 = 36 kWh (129 MJ).
As with the water tank above, some additional discussion needed about heat engines and tank insulation.
Conclusion: Practical and safe, but needs efficient heat engine.
Below: The Crescent Dunes Solar Plant uses molten salt for energy storage.

Heat engines
Heat engines have a very low efficiency. The second law of thermodynamics limits the maximum theoretical efficiency of a heat engine to the following formula: MaxEff = 1 - Tc / Th, (Tc = temperature of the cold side in Kelvin, Th = temperature of the hot side in Kelvin). In other words, the heat storage that powers the engine should be as hot as possible for optimum efficiency.
Powering an ideal heat engine from the tanks above is limited by a maximum efficiency of: MaxEff, water tank = 1 - 293 / 353 = 17% (0.17). MaxEff, salt tank = 1 - 293 / 523 = 44% (0.44). But no heat engine is ideal, so they will all lower the overall efficiency further.
Steam engines were very common a few hundred years ago, having an efficiency of around 10%. They were later replaced with steam turbines with an efficiency of up to 60%, which are now used in most power plants such as nuclear power.
The Stirling engine is used in scenarios where the hot/cold temperature difference is low, so it would be suited for the heated water tank above. It has an efficiency of around 20%.
The thermoelectric cell (also called Peltier element) converts heat directly into electricity, at an efficiency of around 6%.
Combined energy storage
Instead of separating the house heating storage from the electric storage, maybe they can be combined into one system, commonly called a Micro combined heat and power system, or micro-CHP.
The proposed system would have a 1000 L hot water tank, at 80 C when fully heated. It would be heated with a boiler burning firewood. Instead of using standard water radiators to heat the house, the hot water from the tank would flow into a heat engine, and the wasted heat from it would heat the house.
On average the electrical needs are 10% of the heating needs, so the heat engine needs to have at least 10% total efficiency. As calculated above, an ideal engine running at 80 C water would have 17% efficiency. That's an ideal engine, and no such engine exists in real life. For this to work the real engine has to be 10/17 = 60% of an ideal engine. Neither the Stirling engine or thermoelectric cell are good enough.
Conclusion: No heat engine good enough to make it work.
Molten salt thermoelectric generator
Imagine the molten salt tank from above, this time 500 L in size. 120 kWh of stored heat at 250 C. A duct runs through the center of the tank, where the sides are covered with thermoelectric cells with heat sinks on them. A fan blows cold air through the duct to generate hot air and electricity. The hot air can heat the home or in the summer be directed to the outside.
The system would operate at an efficiency of around 44% * 6% = 2.5% for the electrical generation, in other words it has 3 kWh of stored electricity and 117 kWh of stored heat.
Conclusion: A very inefficient way of storing electricity.
Woodgas
Generating combustible gas from firewood was a popular alternative in Sweden during WW2, when gasoline wasn't readily available. A wood gasifier unit was attached to the back of vehicles and the generated gas (mostly carbon-monoxide and hydrogen) could be fed directly to the gasoline engine without modifications. The gasifier was filled with wood chips, turned on, and by the use of pyrolysis generated the woodgas.
Below: a gengas (woodgas) unit in Sweden during the 1940s.

Around 75% of the firewood energy is converted into wood gas in the gasifier. Practical tests have shown that on such a converted vehicle, 1000 kg of fire wood equals 365 liters of gasoline in similar driving conditions. Typically you would use wood from your own forest, but even if you bought the wood the price per traveled km would be three times cheaper running it on wood compared to gasoline.
The gasifier can power an off the shelf genset directly (gasoline powered electrical generator), with practical tests showing a consumption of 1.1 kg wood per kWh produced electricity.
The downside of a wood gasifier is that the produced carbon-monoxide is very toxic, that it needs frequent maintenance/cleaning, and that the produced gas needs a somewhat complicated filter to remove tar and other substances that could clog up the engine. Carbon-monoxide can't be stored, and the gasifier cant be started/stopped on demand, so the most practical scenario is to load up and run the gasifier for a few hours once a day and have the genset charge batteries, which then supply the electricity on demand to appliances.
The upside is that a wood gasfier will greatly simplify my energy system. Fire wood would be used both for heating and generating electricity, with solar panels to boost the electricity when sunshine is available. All I need is big stacks of fire wood from my own forest.
Another interesting idea is the woodgas SOFC, which is using a high temperature fuel cell to convert the woodgas directly to electricity, eliminating the need for a genset. Research is currently under way on this.
The GEK gasifier is a project aimed for developing countries, with free plans available to build a wood gasifier.
The Vedbil project shows how to construct a gasifier for car use, with a diary outlining the author's travels with such a car.
Conclusion: certainly looks interesting, will investigate more.
Back to batteries
So maybe batteries are the only practical solution right now. I've only mentioned lead acid batteries so far, but the auto industry is pushing hard to get the price for Li-ion batteries down, and this may result in cheaper and better batteries for home storage as well.
The electric car maker Tesla is now selling their Power Wall for $5,500, which is a 14 kWh Li-ion battery. The same money will get you a 42 kWh lead acid battery, which is a lot better to have in emergency situations, so lead acid still has an edge on Li-ion for home use. However, for day-to-day long term use these alternatives are quite similar, since a Li-ion battery can be discharged to 0%, while a lead acid degrades quickly if it is discharged below 66%.
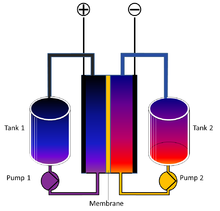 Research is going on to perfect the redox flow battery, a new battery type that would fit home storage very well. It has two tanks of liquid passing by a membrane where charging and discharging takes place, similar to the proton membrane in a fuel cell. To add battery capacity you simply fill up the tanks with more liquid.
Research is going on to perfect the redox flow battery, a new battery type that would fit home storage very well. It has two tanks of liquid passing by a membrane where charging and discharging takes place, similar to the proton membrane in a fuel cell. To add battery capacity you simply fill up the tanks with more liquid.
Then there is the option to refurbish lead acid batteries when they have worn out. Wouldn't it be great if you could take apart the battery, collect the sulfuric acid, melt down the lead, then cast some new plates, and put it all back together again?
It turns out that this is messy, toxic and complicated work. It is a simple technology in theory, so to make a battery that works is easy. But to reach the level of performance you get from a factory made battery, you need complicated machines and you don't want to be involved with handling the components.
The overall conclusion is that I will get some more batteries, perhaps double my capacity, try to be a bit thrifty with the electricity use, and hope that when the batteries are worn out 10 years later there will be much cheaper alternatives available that I can switch to. Meanwhile, I will do some experiments with wood gas, and read up on ways to build lead acid batteries using the Plante process.
Below: Refurbishing batteries in Nepal. Not recommended if you want a long life.
